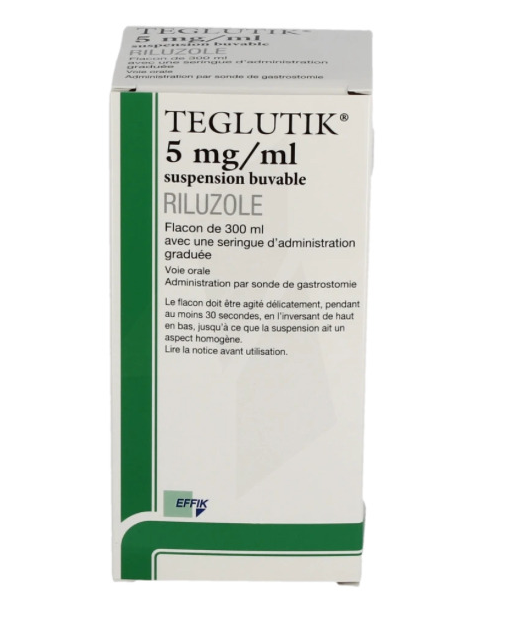
TEGLUTIK (Riluzol) 5mg/ml 300 ml
TEGLUTIK (Riluzol) 5mg/ml 300 ml
TEGLUTIK 5 mg/ml oral suspension
Posology
Method of administration
The drug is not used during pregnancy, breast-feeding and for children. It is prescribed with caution for liver and kidney diseases.
Manufacturer Italfarmaco EFFIK Italy
Ask a question or inquire about delivery to your country