Tipanat Trifluridine + Tipiracil (Lonsurf®) 20 tab
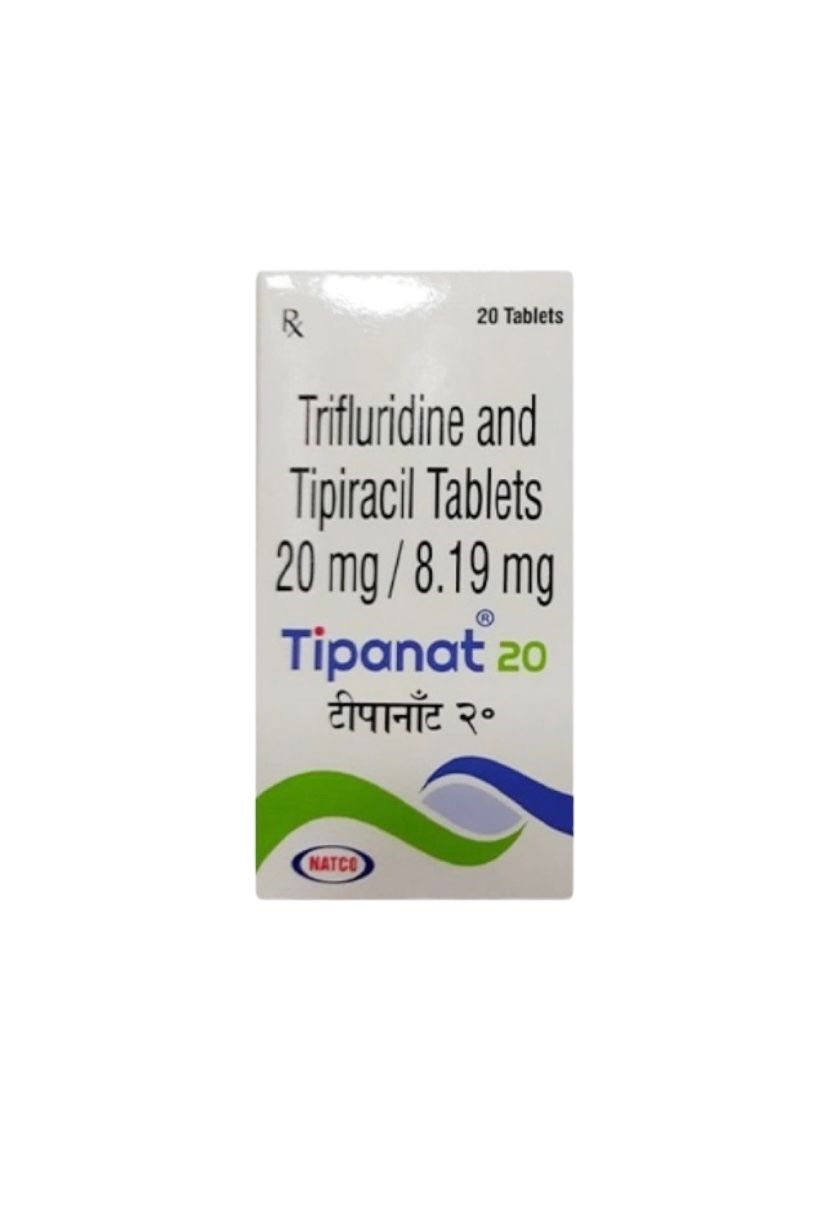
Tipanat Trifluridine + Tipiracil (Lonsurf®)
Manufacturer Information
- Manufacturer: Natco Pharma Ltd., India
- Therapeutic class: Antineoplastic agent
- Reference product: Lonsurf® (Taiho Pharmaceutical Co., Ltd., Japan)
- Bioequivalence: Tipanat is a generic equivalent of Lonsurf, meeting international pharmaceutical quality standards.
Available in: 15 mg / 6.14 mg and 20 mg / 8.19 mg
Tipanat is an oral anticancer medication developed by Natco Pharma Ltd. (India), containing a fixed-dose combination of Trifluridine and Tipiracil Hydrochloride.
It is therapeutically equivalent to Lonsurf® (by Taiho Pharmaceutical Co., Ltd., Japan) and used for the treatment of metastatic colorectal cancer and metastatic gastric or gastroesophageal junction cancer in adults who have previously received standard chemotherapy regimens.
Active Ingredients
Each film-coated tablet contains:
- Trifluridine (FTD): 15 mg or 20 mg
- Tipiracil Hydrochloride (TPI): 6.14 mg or 8.19 mg
Mechanism of Action
Tipanat combines two synergistic components:
- Trifluridine — a nucleoside analog that incorporates into DNA, interfering with tumor cell replication and leading to cell death.
- Tipiracil Hydrochloride — an inhibitor of the thymidine phosphorylase enzyme, which prevents the breakdown of trifluridine, thereby enhancing its bioavailability and prolonging its anticancer activity.
This dual mechanism enables sustained suppression of tumor growth, even in patients resistant to conventional fluoropyrimidine-based therapies.
Therapeutic Indications
Tipanat is indicated for the treatment of adult patients with:
- Metastatic colorectal cancer (mCRC) previously treated with or not candidates for fluoropyrimidine-, oxaliplatin-, irinotecan-, anti-VEGF-, and/or anti-EGFR-based therapies.
- Metastatic gastric cancer or gastroesophageal junction adenocarcinoma after at least two prior lines of systemic treatment.
Dosage and Administration
- Recommended dose: 35 mg/m² (based on the trifluridine component) twice daily, taken within 1 hour after meals (typically breakfast and dinner).
- Treatment cycle: 5 days of dosing, 2 days off, followed by another 5 days of dosing (Days 1–5 and 8–12 of each 28-day cycle).
- Tablets must be swallowed whole, without crushing or chewing.
- Blood counts and clinical parameters should be checked before each cycle.
Dose adjustments may be required based on toxicity and individual tolerance as determined by the treating oncologist.
Contraindications and Warnings
- Hypersensitivity to trifluridine, tipiracil, or any excipient.
- Severe bone marrow suppression (neutropenia, anemia, thrombocytopenia).
- Severe hepatic impairment.
- Pregnancy or breastfeeding.
- Effective contraception is required during treatment and for at least 6 months after completion.
Possible Adverse Effects
Commonly observed side effects include:
- Hematologic: neutropenia, anemia, thrombocytopenia
- Gastrointestinal: nausea, vomiting, diarrhea, decreased appetite
- General: fatigue, weakness, fever, risk of infections
Other side effects may include abdominal pain, headache, or mild liver enzyme elevations.
Adverse events are generally manageable under medical supervision.
Important Note
Tipanat should be used only under the supervision of an oncologist.
Not intended for self-medication.
Periodic blood tests and medical evaluations are mandatory during therapy.
Ask a question or inquire about delivery to your country